
“Forever chemicals” is a colloquial term for per- and polyfluoroalkyl substances (PFAS), a large group of man-made chemicals that have been widely used in various industrial applications and consumer products since the 1940s. read here to learn more about them.
Forever chemicals are characterized by their extremely strong carbon-fluorine bonds, which make them resistant to heat, water, and oil, as well as degradation in the natural environment.
This durability, while valuable for certain uses, means that PFAS can persist in the environment and human bodies for long periods, leading to concerns about their potential health and environmental impacts.
Forever chemicals: What are PFAS?
Per- and polyfluoroalkyl substances (PFAS) are a large family of synthetic chemicals that contain multiple fluorine atoms attached to an alkyl chain.
- Known for their strong carbon-fluorine bonds, PFAS are exceptionally resistant to heat, water, oil, and stains, as well as degradation in the environment.
- This resistance to breakdown, combined with their widespread use since the 1940s, has led to PFAS being referred to as “forever chemicals.”
Uses of Forever chemicals
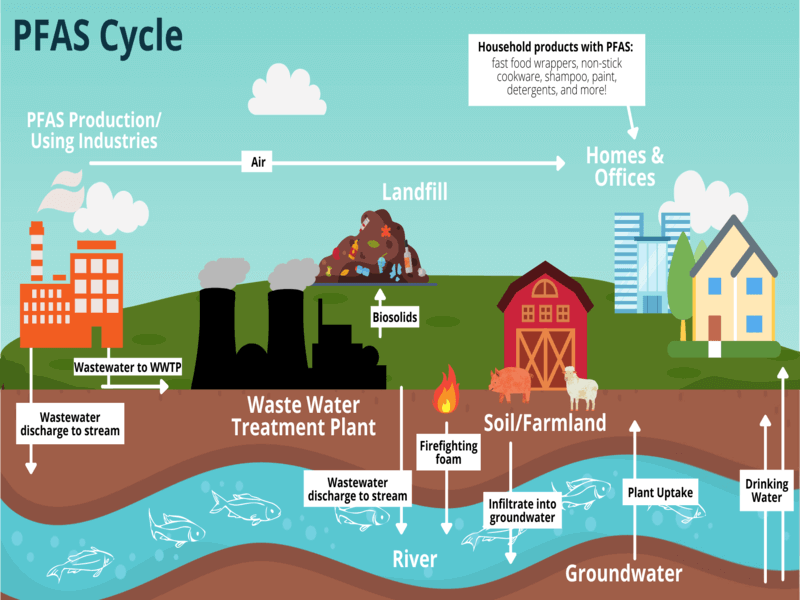
PFAS have been used in a wide range of products due to their water- and grease-resistant properties. Common applications include:
- Non-stick cookware (e.g., Teflon)
- Water-repellent clothing
- Stain-resistant fabrics and carpets
- Food packaging (e.g., microwave popcorn bags, fast-food wrappers)
- Firefighting foams (especially those used at airports and military bases)
- Cosmetics and personal care products (e.g., shampoo, dental floss)
Environmental and Health Concerns

The persistence of forever chemicals in the environment and their ability to accumulate in the human body have raised significant health and ecological concerns.
Studies have linked exposure to certain PFAS compounds to a range of health issues, including:
- Increased cholesterol levels
- Changes in liver enzymes
- Decreased vaccine response in children
- Increased risk of high blood pressure or preeclampsia in pregnant women
- Changes in birth weight
- Increased risk of kidney or testicular cancer
Because of their persistence, PFAS can travel long distances in water and air, leading to widespread contamination of soil, groundwater, and surface water.
This contamination can affect drinking water supplies, agricultural products, and wildlife.
Remediation Efforts
Removing PFAS (per- and polyfluoroalkyl substances) from the environment, particularly from water supplies, is challenging due to their chemical stability and resistance to degradation.
- However, several technologies have been developed and are in use for treating water contaminated with PFAS.
- These methods vary in their effectiveness, cost, and practicality depending on the specific PFAS compounds present and the context of the contamination.
Key methods include:
Granular Activated Carbon (GAC) Filtration
- Activated carbon filters work by adsorbing PFAS compounds onto the surface of the carbon granules as water passes through the filter.
- Pros: Effective for removing a wide range of PFAS compounds; widely used due to its simplicity and effectiveness.
- Cons: Requires regular replacement of the carbon media; less effective for shorter-chain PFAS compounds.
Ion Exchange Resins
- Water passes through a bed of resin beads that have sites for ion exchange. PFAS compounds are attracted to and held by these sites, removing them from the water.
- Pros: Can be more effective than GAC for some PFAS compounds; resin can sometimes be regenerated and reused.
- Cons: Resin beds also need maintenance and eventual replacement; potential for PFAS to break through if the resin becomes saturated.
High-Pressure Membranes
- Reverse osmosis (RO) and nanofiltration (NF) are the most common types used for PFAS removal.
- These processes use a semi-permeable membrane to remove PFAS molecules from water by size exclusion as water is forced through the membrane under high pressure.
- Pros: Highly effective for removing a wide range of PFAS compounds, including shorter-chain PFAS.
- Cons: High energy and maintenance costs; generates a concentrated stream that must be treated or disposed of.
Advanced Oxidation Processes (AOPs)
- AOPs use strong oxidants, often combined with UV light, to break down PFAS compounds into less harmful substances.
- Pros: Can potentially destroy PFAS molecules rather than just removing them from water.
- Cons: High operational costs; effectiveness varies based on the PFAS compound and water chemistry; may produce harmful byproducts.
Regulatory Actions
In response to growing concerns about the health and environmental risks of PFAS, governments and international bodies have begun to take action. These actions include:
- The United States Environmental Protection Agency (EPA) issued advisories and took steps toward regulating PFAS in drinking water, as well as researching their effects and remediation methods.
- The European Union restricted the use of certain PFAS chemicals and set limits for PFAS in drinking water.
- Companies faced lawsuits and increased pressure to phase out the use of PFAS in products and to take responsibility for environmental contamination.
PFASs and Stockholm convention
The Stockholm Convention on Persistent Organic Pollutants was adopted by the Conference of Plenipotentiaries on 22 May 2001 in Stockholm, Sweden.
- The Convention entered into force on 17 May 2004.
- PFHxS, PFOA and PFOS are the three subgroups of PFASs currently listed under the Stockholm Convention as industrial POPs.
Perfluorooctane sulfonic acid (PFOS)
- PFOS listed in Annex B (restriction) since 2009 is both intentionally produced and an unintended degradation product of related anthropogenic chemicals.
- The current intentional use of PFOS is widespread and includes electric and electronic parts, such as fire-fighting foam, photo imaging, hydraulic fluids, and textiles.
- PFOS is still produced in several countries. Its acceptable uses include as an active ingredient in insect bait to control leaf-cutting ants, in closed-loops systems in metal plating and as fire-fighting foam.
Perfluorooctanoic acid (PFOA)
- Perfluorooctanoic acid (PFOA) is listed in Annex A (elimination) since 2019. PFOA are used widely to produce non-stick kitchenware and food processing equipment.
- The unintentional formation of PFOA is created from inadequate incineration at moderate temperatures of municipal solid waste within inappropriate or open burning facilities.
In 2019, at the Second session of the International Conference on Chemicals Management (ICCM2), SAICM stakeholders identified managing PFASs and the transition to safer alternatives as an issue of concern.
- The 10th meeting of the Conference of the Parties in 2022 listed perfluorohexane sulfonic acid (PFHxS) as widely used in fire-fighting foam, carpets, and non-stick cookware.
Perfluorocarboxylic acids (PFCAs)
- Perfluorocarboxylic acids (PFCAs) are used in various applications, including coating products, fabric/carpet protectors, textile impregnation agents and firefighting foams a candidate POPs proposed for listing under the Stockholm Convention.
Why in the news?
Numerous articles about PFAS being found in various environments and products are being published.
- Potentially toxic chemicals called PFAS (perfluoroalkyl and poly-fluoroalkyl substances) are found in surface and groundwaters around the world at levels much higher than many international regulators allow, a new study found.
- Many popular US bandage brands contain alarming levels of toxic PFAS “forever chemicals”, new research suggests, raising questions about the products’ safety.
- A recent study by IIT Madras reveals the pervasive presence of ‘forever chemicals’, pre- and polyfluoroalkyl substances (PFAS) – in Buckingham Canal, Adyar River and Chembarambakkam Lake.
PFAS in Chennai Lakes
The study findings revealed high levels of PFAS in groundwater samples from Chennai.
- While groundwater samples were collected from in and around the Perungudi dumpsite, surface-level samples were taken from different points along the Buckingham Canal, Adyar River, and Chembarambakkam Lake.
- Samples from a water treatment plant (WTP) near the lake were also tested. All the samples were quantified for eight target PFAS.
- It was also found that the concentrations of all eight target PFAS increased in the treated water of the water treatment plant, compared to the raw water.
- The study notes that this is likely due to unidentified precursors that transform into more stable PFAS end products during treatment.
Industrial emissions, untreated domestic wastewater discharge, and open dump sites have been suspected to be significant sources of contamination, highlighting the need for further investigation to assess the extent of PFAS contamination in Chennai fully.
Conclusion
The issue of “forever chemicals” is a complex environmental and public health challenge that requires coordinated action from governments, industry, and communities.
While the properties of PFAS have led to valuable technological developments, their persistent nature poses risks that necessitate careful management, regulation, and efforts to find safer alternatives.
Related articles:
- Endocrine-disrupting chemicals (EDCs)
- Groundwater contamination in India
- Arsenic contamination in India
-Articles by Swathi Satish





Leave a Reply