
Sir Chandrasekhara Venkata Raman, more commonly known as CV Raman, was a pioneering Indian physicist whose work in the field of light scattering earned him the 1930 Nobel Prize in Physics. Read here to learn more about his life.
National Science Day is celebrated in India on February 28th every year since 1986 to mark the discovery of the Raman Effect by Indian physicist Sir Chandrasekhara Venkata Raman on this day in 1928.
This celebration not only commemorates Raman’s groundbreaking discovery, for which he was awarded the Nobel Prize in Physics in 1930 but also aims to spread the message of the importance of science and its application in the daily life of the people.
The early life of CV Raman
Born on November 7, 1888, in Tiruchirappalli, Tamil Nadu, Raman displayed a prodigious intellect from an early age, finishing his secondary education by the age of 11.
He graduated with a Bachelor of Arts degree from Presidency College, Madras, in 1904, and subsequently completed his Master’s in Physics in 1907.
- Despite beginning his career in the Indian Finance Department as a civil servant, Raman’s passion for science never waned.
- He conducted research at the Indian Association for the Cultivation of Science (IACS) in Kolkata during his spare time, which led to significant discoveries in acoustics and optics.
CV Raman made his first trip to London in 1921, where his reputation in the study of optics and especially acoustics was already known to the English physicists J. J. Thomson and Lord Rutherford.
- Raman’s specialty had been the study of the vibrations and sounds of stringed instruments such as the violin, the Indian veena, and tambura, and two uniquely Indian percussion instruments, the tabla, and the mridangam.
But it was the return trip from London to Bombay aboard the SS Narkunda that would change forever the direction of Raman’s future.
- During the fifteen-day voyage, his restless and probing mind became fascinated with the deep blue color of the Mediterranean.
- Unable to accept Lord Rayleigh’s explanation that the color of the sea was just a reflection of the color of the sky, Raman proceeded to outline his thoughts on the matter while still at sea and sent a letter to the editors of the journal Nature when the ship docked in Bombay.
A short time later Raman was able to show conclusively that the color of the sea was the result of the scattering of sunlight by the water molecules.
- Ironically, it was the same argument that Rayleigh had invoked when explaining the color of the sky – the blue was the result of the scattering of sunlight by the molecules in the air.
Also read: Indian Scientists: From Ancient to Modern Era
Nobel Prize and the Raman Effect
CV Raman’s most celebrated discovery, the Raman Effect, came in 1928. It demonstrated that when light traverses a transparent material, some of the deflected light changes wavelength and amplitude.
- This discovery was groundbreaking because it confirmed the quantum nature of light and was the first strong evidence of the quantum behavior of molecules.
- Raman used a simple apparatus to show that when light passes through a transparent substance, it scatters, and the scattered light contains frequencies not present in the original light, a phenomenon that could not be explained by classical physics.
For this discovery, he was awarded the Nobel Prize in Physics in 1930, becoming the first Asian and the first non-white to receive a Nobel Prize in the sciences.
What is the Raman effect?
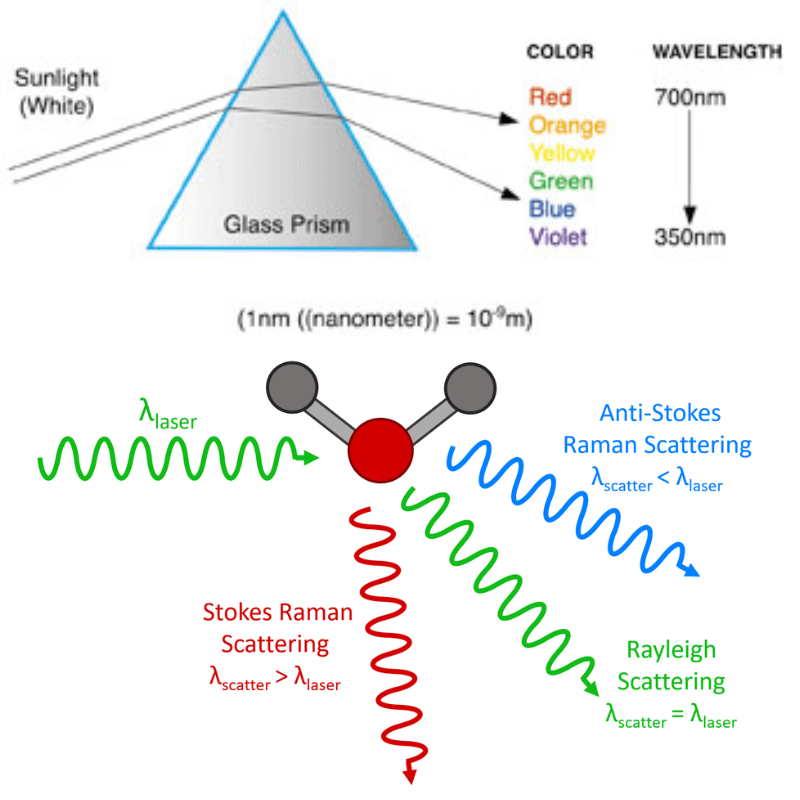
The Raman Effect occurs when light interacts with the molecules of a material, causing a change in the energy and wavelength of the scattered light.
- When monochromatic light (light of a single wavelength, usually from a laser) is directed at a material, most of the light scatters elastically (Rayleigh scattering), meaning it retains its original energy and wavelength.
- However, a small fraction of the light (approximately 1 in 10 million photons) scatters inelastically, either gaining or losing energy in the process. This inelastic scattering is the Raman Effect.
Stokes and Anti-Stokes Scattering
The energy change in the scattered light corresponds to the vibrational energies of the molecules in the material.
- If the scattered light loses energy (shifts to a longer wavelength), it is called Stokes scattering.
- Conversely, if the scattered light gains energy (shifts to a shorter wavelength), it is called Anti-Stokes scattering.
- The difference in energy between the incident and scattered light directly relates to the vibrational energy levels of the molecules in the sample.
The discovery of the Raman Effect was a milestone in experimental physics and quantum theory.
- It provided the first experimental evidence of the quantum nature of light and molecules, supporting the theoretical predictions of quantum mechanics.
- The Raman Effect showed that light-matter interactions could result in the exchange of energy, leading to a deeper understanding of molecular energy levels and the electromagnetic spectrum.
Application of Raman effect
Physics:
In the first seven years after its discovery, the Raman Effect was the subject of more than 700 papers in the scientific literature, mostly by physicists who were using the technique to study the vibration and rotation of molecules and relating those phenomena to the molecular structure.
Chemistry:
By the late 1930s, the Raman Effect had become the principal method of non-destructive chemical analysis for both organic and inorganic compounds.
- The unique spectrum of Raman scattered light for any particular substance served as a “fingerprint” that could be used for qualitative analysis, even in a mixture of materials.
- Raman spectroscopy could be applied not only to liquids but also to gases and solids.
- The use of Raman spectroscopy as a basic analytical tool changed sharply after World War II.
- During the war, infrared spectroscopy was enhanced by the development of sensitive detectors and advances in electronics.
Other applications:
- Material Science: It helps in characterizing materials, understanding their structure, and studying phase transitions.
- Biological Studies: Raman spectroscopy is used in the medical field to diagnose diseases, analyze biochemical changes in cells, and study drug interactions at the molecular level.
- Pharmaceuticals: It assists in drug development and quality control by identifying the molecular composition and crystalline forms of drugs.
- Environmental Science: It is employed in detecting pollutants and analyzing environmental changes.
Academic and Research Contributions
After his Nobel win, Raman’s reputation and influence grew. He served as the director of the Indian Institute of Science (IISc) in Bangalore from 1933 to 1937.
He established the Raman Research Institute in Bangalore in 1948, where he worked until he died in 1970.
He also founded the Indian Journal of Physics in 1926, of which he is the Editor. Raman sponsored the establishment of the Indian Academy of Sciences and has served as President since its inception.
His research at these institutions spanned various domains of physics, including crystal dynamics, musical instruments, and the properties of diamonds.
Legacy
Raman’s legacy is not just in his scientific discoveries but also in his role as a leader in Indian science. He was instrumental in promoting scientific research in India, inspiring generations of scientists.
Despite facing several challenges, including limited resources and recognition from the global scientific community initially, Raman’s perseverance and dedication to science shone brightly. His work laid the groundwork for numerous scientific advancements, including the study of molecular energy levels, chemical analysis techniques, and even the investigation of quantum mechanics.
Sir CV Raman passed away on November 21, 1970, leaving behind a rich legacy of scientific inquiry and discovery. His life and work continue to inspire scientists around the world, underscoring the importance of curiosity, perseverance, and the relentless pursuit of knowledge.
Related article:
-Article by Swathi Satish





Leave a Reply