
The Haber-Bosch process, developed in the early 20th century, revolutionized global agriculture by enabling large-scale ammonia synthesis from atmospheric nitrogen. This ammonia was then used to create synthetic fertilizers, which profoundly impacted food production. Read here to learn more.
Before the Haber-Bosch process, natural nitrogen sources, such as guano and manure, were insufficient to meet global agricultural demands.
This industrial process currently removes around 100 million tonnes of nitrogen from the atmosphere annually, adding 165 million tonnes of reactive nitrogen to soils.
Without this process, meeting global food demand would have been impossible, given that biological nitrogen fixation naturally provides only 100-140 million tonnes of reactive nitrogen per year.
What is Haber-Bosch process?
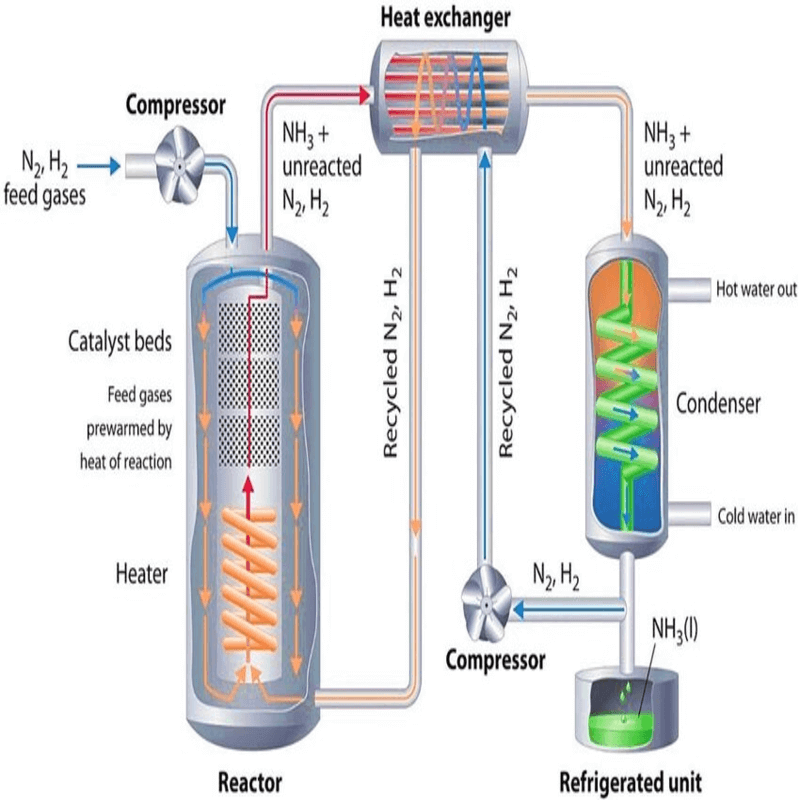
The Haber-Bosch process is a chemical procedure used to synthesize ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂) gases, under high pressure and temperature, using an iron catalyst.
It was developed in the early 20th century by German chemists Fritz Haber and Carl Bosch, and it revolutionized agriculture by enabling the mass production of fertilizers.
Key Components of the Process:
- Raw Materials:
- Nitrogen (N₂): Sourced from the atmosphere (which is 78% nitrogen).
- Hydrogen (H₂): Historically sourced from coal or methane (natural gas) through processes like steam reforming.
- Conditions:
- High Pressure: Around 150-200 atmospheres.
- High Temperature: 400-500°C.
- Catalyst: Typically, an iron-based catalyst, though modern processes may use enhanced materials to increase efficiency.
- Chemical Reaction: It takes place under high temperature and pressure in the presence of the catalyst.

The reaction is exothermic, meaning it releases energy as heat.
Significance:
- Agricultural Impact: The ammonia produced is a key ingredient in nitrogen-based fertilizers, crucial for boosting agricultural productivity and sustaining the global food supply. Before this process, nitrogen fixation was largely dependent on natural, slower processes.
- Industrial Scale: The Haber-Bosch process is still one of the most important industrial chemical processes, producing millions of tons of ammonia annually, mainly for fertilizers.
- Environmental Concerns: While the process has greatly increased food production, it also has environmental downsides. The industrial production of ammonia is energy-intensive and heavily reliant on fossil fuels, contributing significantly to CO₂ emissions.
The Haber-Bosch process is critical for modern agriculture and food security but also represents a challenge in terms of sustainability and environmental impact.
Impact on Agriculture and Society
- Dramatic Increase in Food Supply: The synthetic fertilizers produced using the Haber-Bosch process led to a significant increase in crop yields, contributing to what is often called the “Green Revolution.” This process is credited with a sevenfold rise in the world’s food supply during the 20th century, allowing the global population to grow rapidly without widespread food shortages.
- Industrialization of Agriculture: The availability of cheap synthetic fertilizers enabled the industrial-scale farming that is common today. Countries could now produce much larger quantities of food, helping to feed rapidly growing populations and reducing the risk of famine.
- Geopolitical Implications: By making nitrogen-based fertilizers more accessible, the Haber-Bosch process lessened countries’ reliance on natural nitrogen sources. This altered global trade patterns and lessened competition for limited natural nitrogen deposits.
Environmental Concerns
While the Haber-Bosch process solved one major problem—how to produce enough food for a growing global population—it introduced significant environmental challenges:
- Eutrophication: The widespread use of synthetic nitrogen fertilizers has led to the runoff of excess nitrogen into water bodies, causing eutrophication. This leads to algal blooms, which deplete oxygen levels in water and create dead zones, harming aquatic ecosystems.
- Greenhouse Gas Emissions: The production of synthetic fertilizers requires significant energy, and the agricultural use of these fertilizers has been linked to the release of nitrous oxide (N₂O), a potent greenhouse gas that contributes to climate change.
- Soil Degradation: Overuse of synthetic fertilizers can lead to soil degradation, reducing its fertility over time. The reliance on chemical fertilizers often replaces more sustainable farming practices like crop rotation and organic fertilization, which help maintain soil health.
Conclusion
The Haber-Bosch process has undeniably changed the world by increasing food production and supporting population growth.
However, it has also created environmental challenges that require careful management, particularly around fertilizer use and its impact on ecosystems and climate change. Sustainable agricultural practices are increasingly being promoted to address these concerns while maintaining food security.
Frequently Asked Questions (FAQs)
Q. Why is the Haber process so important?
Ans: The Haber-Bosch process, which converts hydrogen and nitrogen to ammonia, could be one of the most important industrial chemical reactions ever developed. The process made ammonia fertilizer widely available, helping cause a world population boom as yields from agriculture increased rapidly in a short time.
Q. What is the catalyst for the Haber-Bosch process?
Ans: Catalyst: Iron (Fe) Promoter: Molybdenum (Mo)
Related articles:
-Article by Swathi Satish





Leave a Reply